EXCIPIENTS:
Disodium Edetate Hydrate, Clorua benzalkonium (10%),
Polyoxyethylene Hydrogenated Castor Oil 60, Glycerin, Propylene
Glycol, Hydroxyethylcellulose, l-Menthol, dl-Camphor, Sodium
Hydroxide and purified water
ACTIVE INGREDIENT (IN 500ML):
ε-Aminocaproic Acid: 500 mg, Chlorpheniramin Maleate: 15 mg,
Pyridoxine Hydrochloride: 25 mg, Panthenol: 25 mg,
d-α-Tocopherol Acetate: 25 mg, Potassium L-Aspartate: 250 mg,
Taurine (2-Aminoethanesulfonic acid): 250 mg

eyemiru wash
THE NUMBER OF CONFIRMATION DRUG ADVERTISING CONTENT OF MINISTRY OF HEALTH: 0358/XNQC/QLD NOVEMBER 21ST, 2017Being Popular in the Asian market.
Eyemiru Wash is 100% manufactured and packaged in Japan with such nutrious ingredients as Vitamin E, B5, B6, helps wash away dirt after going out, prevent eye diseases after swimming and reduce eye fatigue (Can be used before sleeping).
Eyemiru Wash is used for many types of objects, especially after removing makeup, swimming and dust exposure.
INDICATION&
CONTRAINDICATION
INDICATION:
USE TO WASH YOUR EYES, PREVENT EYE DISEASE (AFTER SWIMMING, BEING INFECTED WITH DUST INTO EYES…)
CONTRAINDICATION:
DO NOT USE FOR THOSE WHO HAVE ALLERGY TO ANY INGREDIENT IN THIS PRODUCT.
1. TALK TO YOUR DOCTOR OR PHARMACIST IN FOLLOWING CASES:
(1) Be under medical treatment or using any other type of eye
drops or eye ointment
(2) May cause allergy for those who have history of allergy to
any drugs
(3) Suffer serious eye aches
(4) Be sensitive to any ingredients in this product.
2. THERE ARE NOT ANY REPORTS ABOUT THE IMPACT OF THE PRODUCT ON PREGNANT AND LACTATION WOMEN.
3. THERE ARE NOT ANY REPORTS ABOUT THE IMPACT OF THE PRODUCT ON THOSE WHO ARE OPERATING
MACHINES, SHIPS AND VEHICLES.
(1) Only use for washing the eyes.
(2) Do not use when the product has changed color or become cloudy.
(3) Can be used for children under adult’s supervison.
(4) Clean makeup and dirt around the eyes before using.
(5) Do not reuse solution for the another eye.
(6) Clean the cup before and after using, then storing in dry condition.
(7) Do not use while wearing contact lens.
(8) Do not use the same cup with others.
STOP USING AND BRINGING THIS INSERT TO WORK ON CONSULTATION WITH DOCTOR OR PHARMACIST IN FOLLOWING CASES:
Rashes, flares, congestion, itching or irritation, swelling, burning, smarting pain occurs in the eye. Or rashes, flares, itching sensation occurs on the skin after use.
(1) Storage at 1 ~ 30oC, protected from direct sunlight.
(2) Shelf life: 24 months from date of manufacture.
(3) Use within 30 days after open the cap. Do no use if product
is expired.
(4)Keep out of reach of children
(5) Do not store the content in another bottle to avoid mistake or
worsening of the quality
(6) Do not share the same product with another person.

WARNINGS

MODE OF APPLICATION
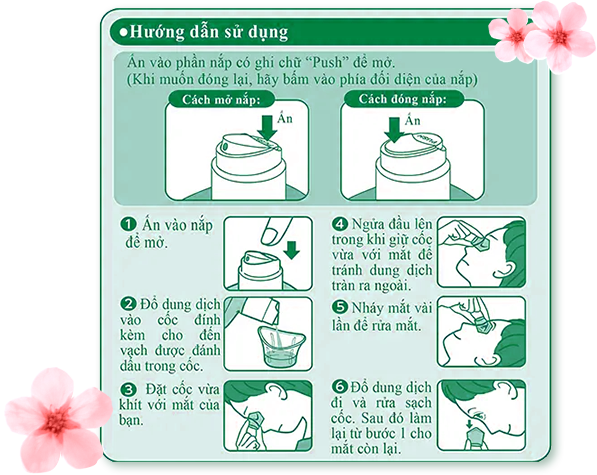
Wash each eye with 5 mL of this product, use 3 to 6 times per day, or follow to doctor or pharmacist’s direction.
No information
The eye drop might cause itchiness, redness in some cases. Do consult doctors and eye professional once you experience these unwanted effects.
No information
READ THE INSTRUCTION CAREFULLY BEFORE USE



